Extracellular vesicles:
Extracellular vesicles (EVs) are small, membrane-bound structures released by cells into their surrounding environment. They play important roles in intercellular communication by carrying various biomolecules, such as proteins, nucleic acids, and lipids, between cells. EVs have gained significant attention in recent years due to their potential as diagnostic and therapeutic tools.
There are different types of extracellular vesicles, but the two most studied types are exosomes and microvesicles. Exosomes are typically smaller, ranging from 30 to 150 nanometers in diameter, and are formed within the endosomal system of cells. Microvesicles, also known as ectosomes or shedding vesicles, are larger, ranging from 100 to 1,000 nanometers, and are formed by the direct budding and shedding of the plasma membrane.
To detect extracellular vesicles, various techniques can be employed. Here are a few commonly used methods:
- Electron microscopy: This technique allows for direct visualization of extracellular vesicles by using an electron microscope. It provides high-resolution images that can reveal the size, morphology, and structure of the vesicles.
- Nanoparticle tracking analysis (NTA): NTA utilizes the Brownian motion of particles to measure the size and concentration of extracellular vesicles in a sample. It involves tracking the movement of individual vesicles under light scattering and analyzing the data to determine their size distribution.
- Flow cytometry: Flow cytometry can be used to analyze and quantify extracellular vesicles based on their size, surface markers, and fluorescence properties. Fluorescently labeled antibodies or markers specific to EVs are used to identify and analyze the vesicles in a flow cytometer.
- Western blotting: Western blotting is a technique that detects specific proteins in a sample. By isolating the proteins from extracellular vesicles and subjecting them to Western blot analysis, you can identify and quantify particular proteins associated with EVs.
- Polymerase chain reaction (PCR): PCR can be used to detect and analyze nucleic acids, such as microRNAs or mRNAs, present in extracellular vesicles. By isolating the RNA or DNA from EVs and performing PCR amplification, you can assess the presence or abundance of specific genetic material.
It’s worth noting that each detection method has its advantages and limitations, and the choice of technique depends on the specific research or diagnostic needs. Additionally, ongoing research is exploring new and improved methods for the detection and characterization of extracellular vesicles
Extracellular vesicles (EVs) have attracted significant attention as impactful diagnostic biomarkers, since their properties are closely related to specific clinical conditions. However, designing experiments that involve EVs phenotyping is usually highly challenging and time-consuming, due to laborious optimization steps that require very long or even overnight incubation durations. (Unlu et al).
In the past few years, the interest in Extracellular Vesicles (EVs) as theranostic tools has significantly increased. These biological particles constitute a very heterogeneous population in the human body, in both origin and size. They range from vesicles of endosomic origin (small EVs, or exosomes, 50–150 nm) to microvesicles (50 nm–1 um) released from the plasma membrane. The heterogeneity of these biological nanoparticles can sometimes pose a challenge in terms of purification and phenotyping.
An established method for the real-time detection of extracellular vesicles is Surface Plasmon Resonance Imaging (SPRi). This technique succeeds in monitoring the label-free binding of EVs to different probes simultaneously. However, as in the case of all evanescent-wave based sensors, SPR cannot distinguish surface binding from local changes in solution refractive index, and it is prone to environmental factors (temperature, vibrations, pH variations). Since EVs are normally purified from plasma or cell culture media, the typical target solutions are highly heterogenous. Therefore, the large variations in refractive index further exacerbate the background noise in SPR measurements.
An example of SPRi detection is from LABION where they isolate EVs from biological fluids, such as human serum, plasma and saliva, to be studied as biomarkers of neurodegenerative and cerebrovascular diseases. The SPRi platform is used succesfully for the detection and characterization of multiple populations of EVs in order to support the diagnosis and to monitor disease progression evaluating the effects of therapeutic and rehabilitative treatments. The iFOUR Dispensing system is used for the preparation of the SPRi microarray where ligands are antibodies selected for the simultaneous and specific detection of multiple subpopulations of EVs coming from neurons, astrocytes, oligodendrocytes, microglia and endothelial cells, in order to compare their levels in samples from healthy subjects and patients in different disease stages.
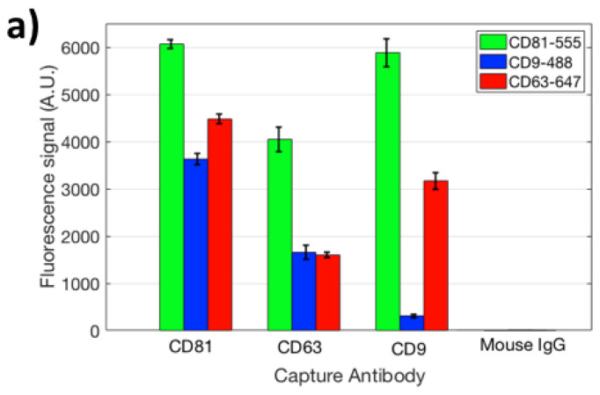
The Interferometric Reflectance Imaging Sensor technology (iRiS) is a new development that addresses limitations of SPRi. This publication gives a good description of its use in EV detection. It can be used to measure shape and size distribution, as shown on Figure A.